Noncanonical function of folate through folate receptor 1 during neural tube formation
Balashova OA, Panoutsopoulos AA, Visina O, Selhub J, Knoepfler PS, Borodinsky LN.
Nat Commun 2024 Feb 22;151:1642. doi: 10.1038/s41467-024-45775-1.
Click here to view article at Nature Communications.
Click here to view article on Pubmed.
Click here to view article on Xenbase.
Neural tube defects are serious and common birth defects that occur when the neural tube fails to close at four weeks of pregnancy. Supplementation with the vitamin B9 folate periconceptionally has shown to decrease the incidence of these defects by unclear mechanisms. Here we show that the interaction of folate with folate receptor 1, known as one of folate uptake systems, is necessary for neural tube formation by regulating cell-cell adhesion in hiPSC-derived neural organoids and in Xenopus laevis embryos independently of its canonical function of vitamin transport system for the participation of folate in metabolism. Instead, they find that folates and folate receptor 1 regulate Ca2+ dynamics during neural plate folding necessary for neural cell apical constriction.
The authors discover folate receptor 1 interacts with CD2AP and both regulate apical endocytosis in neural plate cells and turnover of cell adherens junction component cadherin in opposing manner. This regulation is crucial for the apical constriction of neural plate cells and the reshaping of the neural plate into a tube during neural tube formation. By demonstrating that it is not the metabolic contribution of folate and the transport function of folate receptor 1, but the regulation of downstream targets that are necessary for neural tube formation, this study is paradigm shifting and addresses a long-standing question in the field. Their findings uncover the mechanism by which folate and folate receptor 1 may prevent the occurrence of neural tube defects.
This study motivates the discussion on the mechanisms of folate action important for neural tube formation and it will be of interest to scientists working in many research fields, such as developmental biology, cell biology, neuroscience, nutrition, and medicine.
Abstract
Folate supplementation reduces the occurrence of neural tube defects (NTDs), birth defects consisting in the failure of the neural tube to form and close. The mechanisms underlying NTDs and their prevention by folate remain unclear. Here we show that folate receptor 1 (FOLR1) is necessary for the formation of neural tube-like structures in human-cell derived neural organoids. FOLR1 knockdown in neural organoids and in Xenopus laevis embryos leads to NTDs that are rescued by pteroate, a folate precursor that is unable to participate in metabolism. We demonstrate that FOLR1 interacts with and opposes the function of CD2-associated protein, molecule essential for apical endocytosis and turnover of C-cadherin in neural plate cells. In addition, folates increase Ca2+ transient frequency, suggesting that folate and FOLR1 signal intracellularly to regulate neural plate folding. This study identifies a mechanism of action of folate distinct from its vitamin function during neural tube formation.
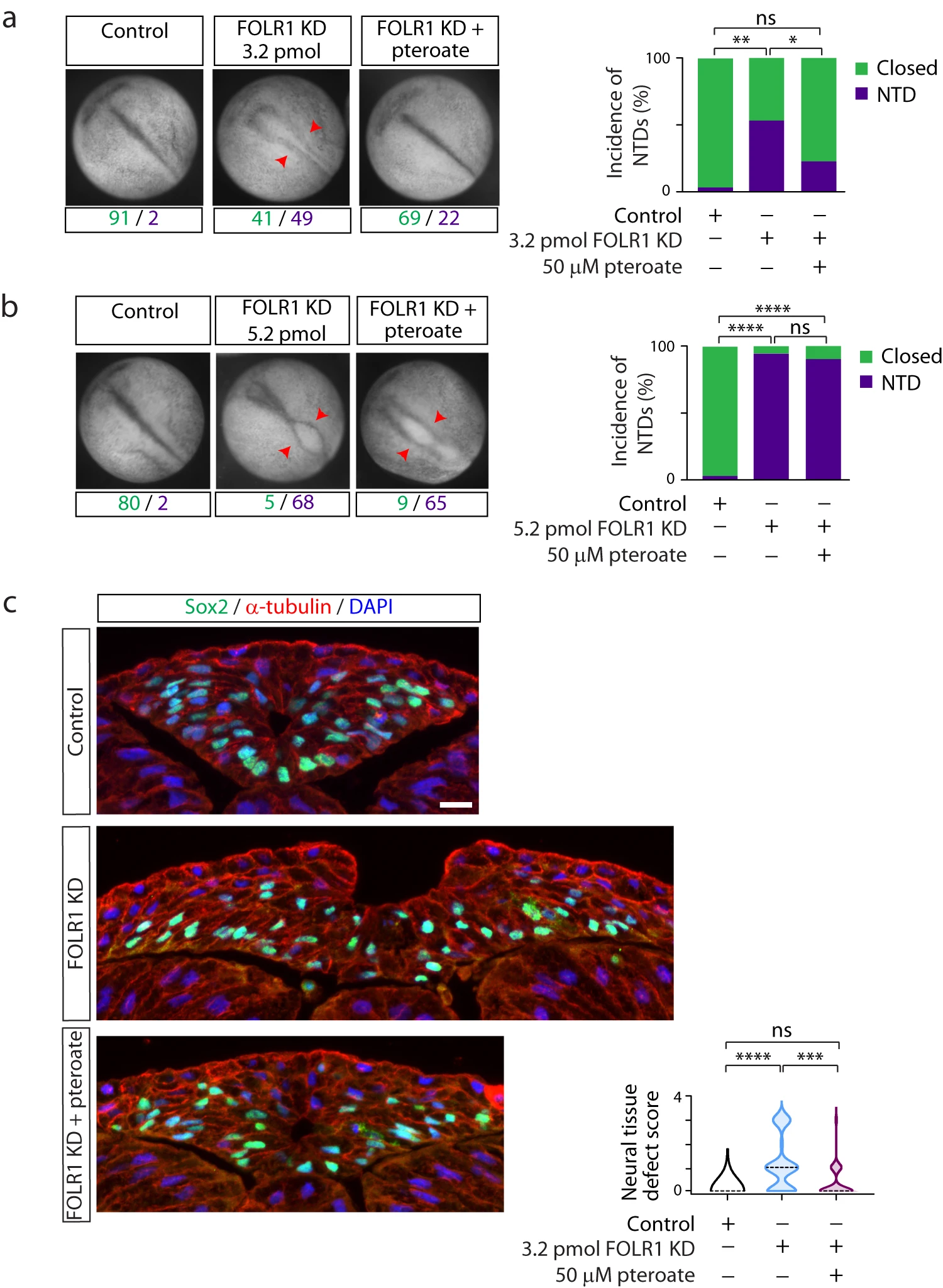
Fig. 2: FOLR1 is necessary for neural tube formation in Xenopus laevis embryos through a non-metabolic mechanism. Two-cell stage Xenopus laevis embryos were microinjected with 3.2 (a, c) or 5.2 (b) pmol Control-MO (Control) or FOLR1-MO (FOLR1 KD) per embryo and incubated with saline or 50M pteroate until the neural tube closed in control embryos, when they were fixed, photomicrographed (a, b), sectioned and processed for immunostaining (c). a, b Examples of embryos in each group. Arrowheads indicate open neural tube (neural tube defect, NTD). Numbers are embryos presenting open (NTD, purple) or closed (green) neural tube. Bar graphs represent proportion of phenotypes in each group. c Examples of immunostained transverse sections of the neural tissue; n of embryos sectioned was 25, 39 and 42 in Control, FOLR1 KD, and FOLR1 KD+pteroate groups, respectively, in N=3 independent experiments. Scale bar is 20m. Graph shows distribution of neural tissue defect score per group, median is indicated by dashed line. In (ac), *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, one-way ANOVA, Tukey post-hoc multiple comparison test (a, b), or Kruskal-Wallis, Dunns multiple comparison test (c). Source data are provided as a Source Data file.
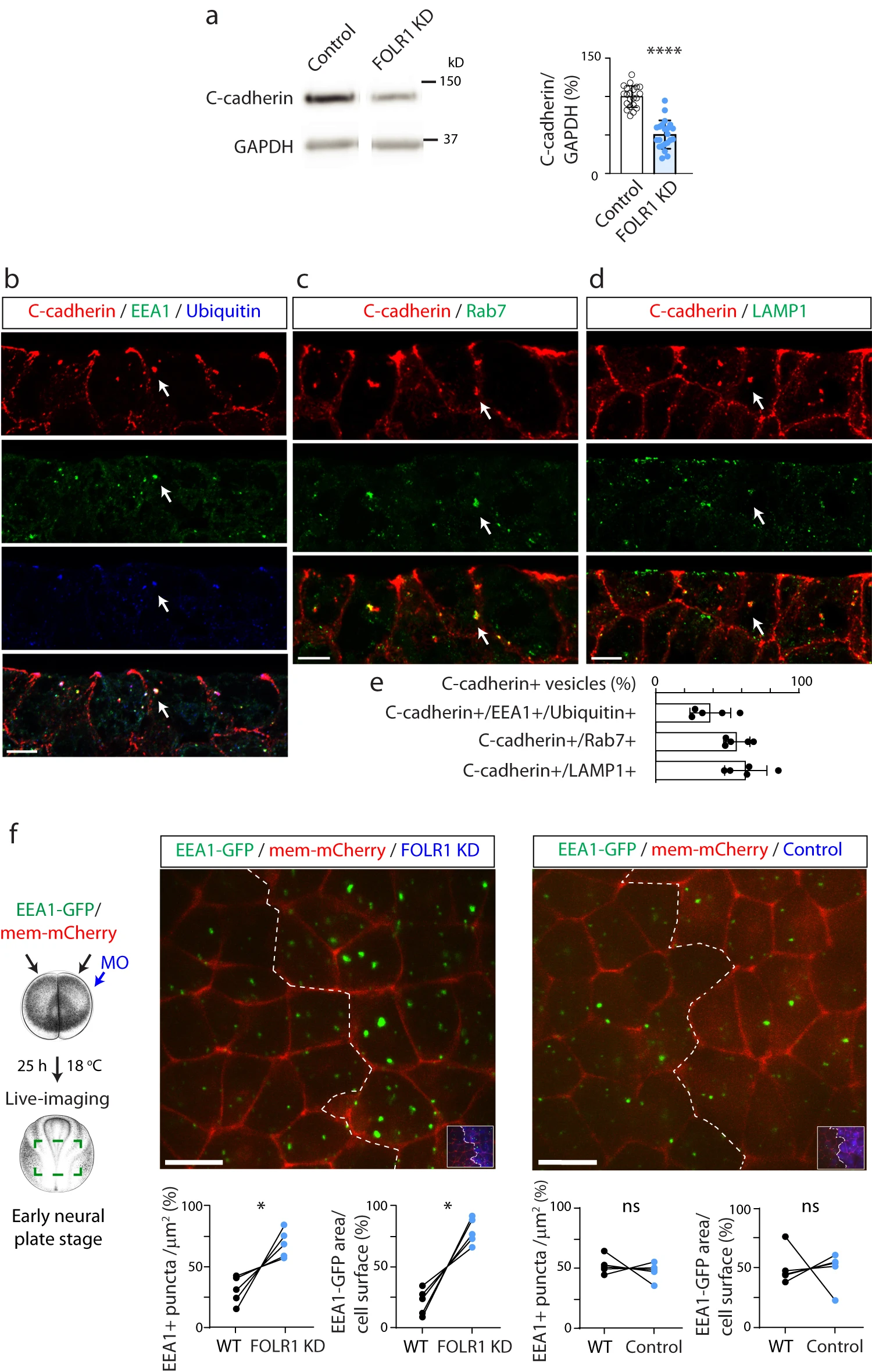
Fig. 4: FOLR1 regulates C-cadherin protein level and endocytosis from apical neural plate cell membrane. a Two-cell stage Xenopus laevis embryos were bilaterally microinjected with 1.6pmol Control-MO (Control) or FOLR1-MO (FOLR1 KD) per blastomere and allowed to grow until they reached mid-neural plate stages (stage 1517) when neural plate was dissected and processed for Western blot assays. Example of Western blot assay. Graph shows individual and meanSD percent of optical density (OD) for C-cadherin immunoblot band normalized with GAPDH protein band OD and compared to controls. Two-tailed ratio t-test, n=42 and 44 neural plates for Control and FOLR1 KD groups, respectively, N=8 independent experiments. be Neural plate stage Xenopus laevis embryos were fixed, sectioned and processed for immunostaining. bd Examples of immunostained neural plate. Arrows indicate colocalization of C-cadherin with early endosomal marker (EEA1) and ubiquitin (b), late endosomal marker (Rab7, c) and lysosomal marker (LAMP1, d). Scale bar, 10m. e Graph shows meanSD proportion of C-cadherin vesicles colocalizing with the indicated markers. Number of cells analyzed were 104, 52, 65 from immunostained samples in (b, c and d), respectively, from N=5 embryos. f Two-cell stage Xenopus laevis embryos were bilaterally microinjected with hEEA1-GFP mRNA and membrane mCherry and unilaterally microinjected with 2.63.2pmol FOLR1-MO (FOLR1 KD) or Control-MO (Control) per blastomere along with fluorescent tracer and allowed to develop until they reached early neural plate stages (stage 13-14), when they were time-lapse imaged with an acquisition rate of 1 frame/6min. Schematic shows experimental design. Xenopus illustrations Natalya Zahn (2022) obtained from Xenbase (www.xenbase.org RRID:SCR_003280), released under a Creative Commons Attribution-NonCommercial 4.0 License (CC BY-NC)68. Images are maximum intensity projection of single time frame. Dashed lines indicate border between wild-type (WT) and MO-injected neural plate. Insets show neural plate injected side with tracer in blue. Graphs show distribution between both halves of the neural plate (in %) of the number of EEA1-GFP vesicles and area fraction of labeled endosomes per neural plate cell surface. Two-tailed paired t-test; ns: not significant, n=30 cells analyzed in each group, N=5 embryos per group. Scale bar, 20m. In a, f, *p<0.05, ****p<0.0001. Source data are provided as a Source Data file.
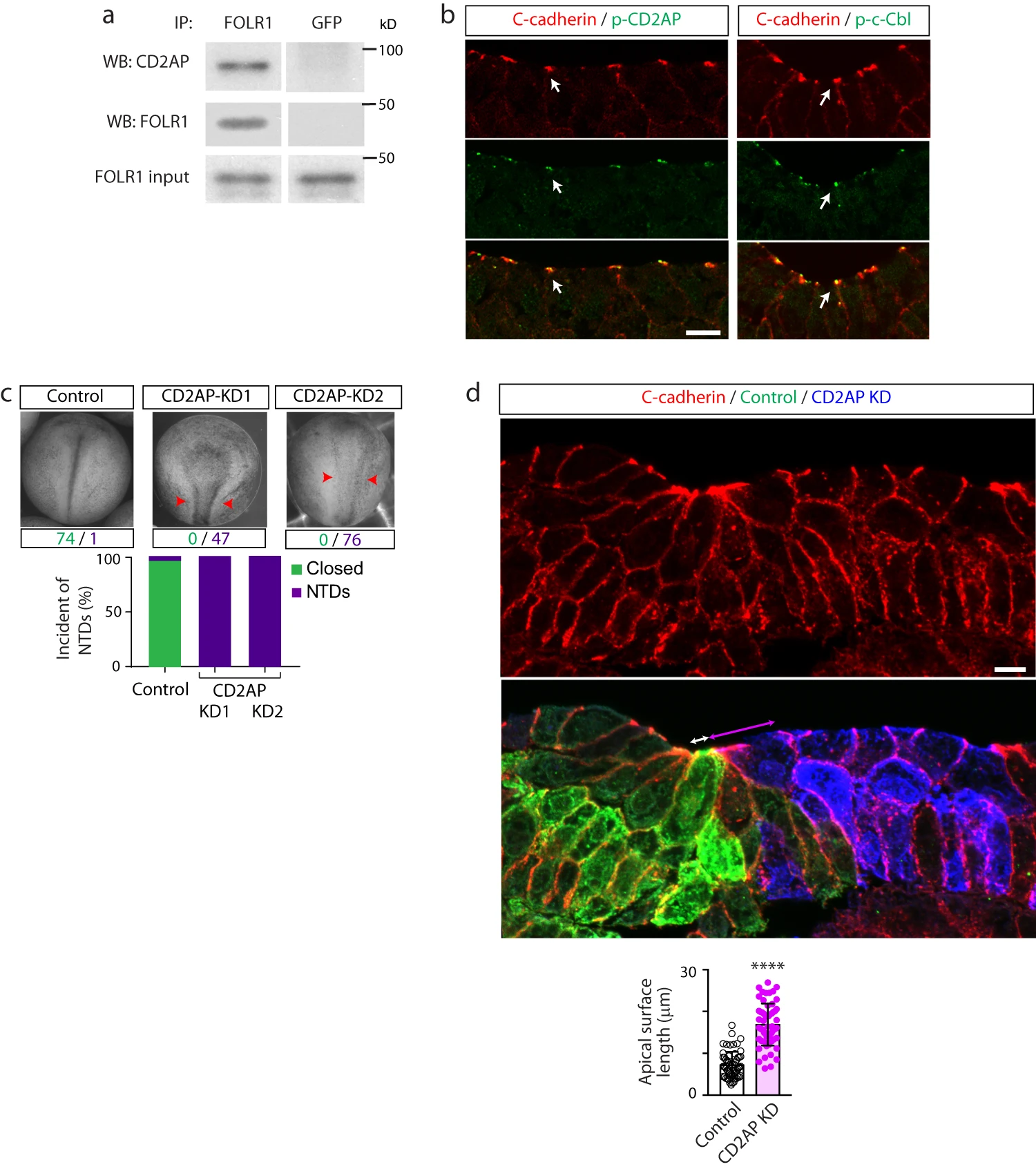
Fig. 6: CD2AP interacts with FOLR1 and is necessary for apical constriction and neural tube formation. a Neural plate stage Xenopus laevis embryos were processed for co-immunoprecipitation (IP) assays. Example of Western blot assay from immunoprecipitates with FOLR1 or GFP (control) antibodies and probed for CD2AP or FOLR1. Similar results were observed in N=3 independent experiments. b Neural plate stage Xenopus laevis embryos were fixed and processed for immunostaining. Images are transverse single z-sections of immunostained neural plate showing apical colocalization of phospho-CD2AP (p-CD2AP) and p-c-Cbl with C-cadherin. Scale bar, 10m. Similar results were observed in N=3 independent experiments. c Two-cell stage Xenopus laevis embryos were bilaterally microinjected with 9.9pmol of Control-morpholino (Control, Control-MO), 2.6pmol CD2AP-MO1 (CD2AP KD1) or 7.49.9pmol CD2AP-MO2 (CD2AP KD2/KD) per blastomere until neural tube closed in control embryos, when they were fixed and photomicrographed. Examples of whole embryos in each group. Arrowheads indicate open neural tube (neural tube defect, NTD). Numbers are embryos presenting closed (green) or open (purple, NTD) neural tube. Bar graph represents proportion of phenotypes in each group. d Two-cell stage Xenopus laevis embryos were unilaterally microinjected with 9.9pmol Control-MO (Control) and 7.49.9pmol CD2AP-MO2 (CD2AP KD) along with GFP and mCherry mRNA, respectively, and allowed to develop until they reached mid-neural plate stages, when they were fixed and processed for immunostaining. Image is a transverse section of the neural plate, immunostained for GFP (Control), mCherry (CD2AP KD) and C-cadherin. Double arrows indicate apical surface length of medial superficial Control (white) and CD2AP KD (magenta) neural plate cells. Scale bar, 20m. Graph shows individual and meanSD apical length of superficial neural plate cells per embryo, n of cells=75 in each half of the neural plate, N of embryos=4. ****p<0.0001, two-tailed paired t-test. Source data are provided as a Source Data file.
Adapted with permission from Springer Nature on behalf of Nature Communications: Balashova et al. (2024). Noncanonical function of folate through folate receptor 1 during neural tube formation. Nat Commun 2024 Feb 22;151:1642. doi: 10.1038/s41467-024-45775-1.
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
Last Updated: 2024-04-30